Bin Hong/Linzhuan Wu’s group: The cytochrome P450 catalyzing C-S bond formation in S-heterocyclization of chuangxinmycin biosynthesis
Source: IMB
time:2021-05-25
Views:
Bin Hong’s group and Linzhuan Wu’s group collaboratively published a article entitled “The cytochrome P450 catalyzing C-S bond formation in S -heterocyclization of chuangxinmycin biosynthesis” in Angewandte Chemie International Edition on July 5, 2021.
Cytochrome P450 monooxygenases (P450s) are well known to convert C-H bonds into C-O (hydroxylation or epoxidation), C-N (amination or nitration) and C-C bonds in both framework construction and structural modification of microbial secondary metabolite biosynthesis. Moreover, a few P450s are suggested to be responsible for C-S bond formation. However, biochemical and structural biology studies are still lacking to confirm the biological functions of these P450s and to elucidate the underlying mechanism(s) in these C-S bond formation reactions.
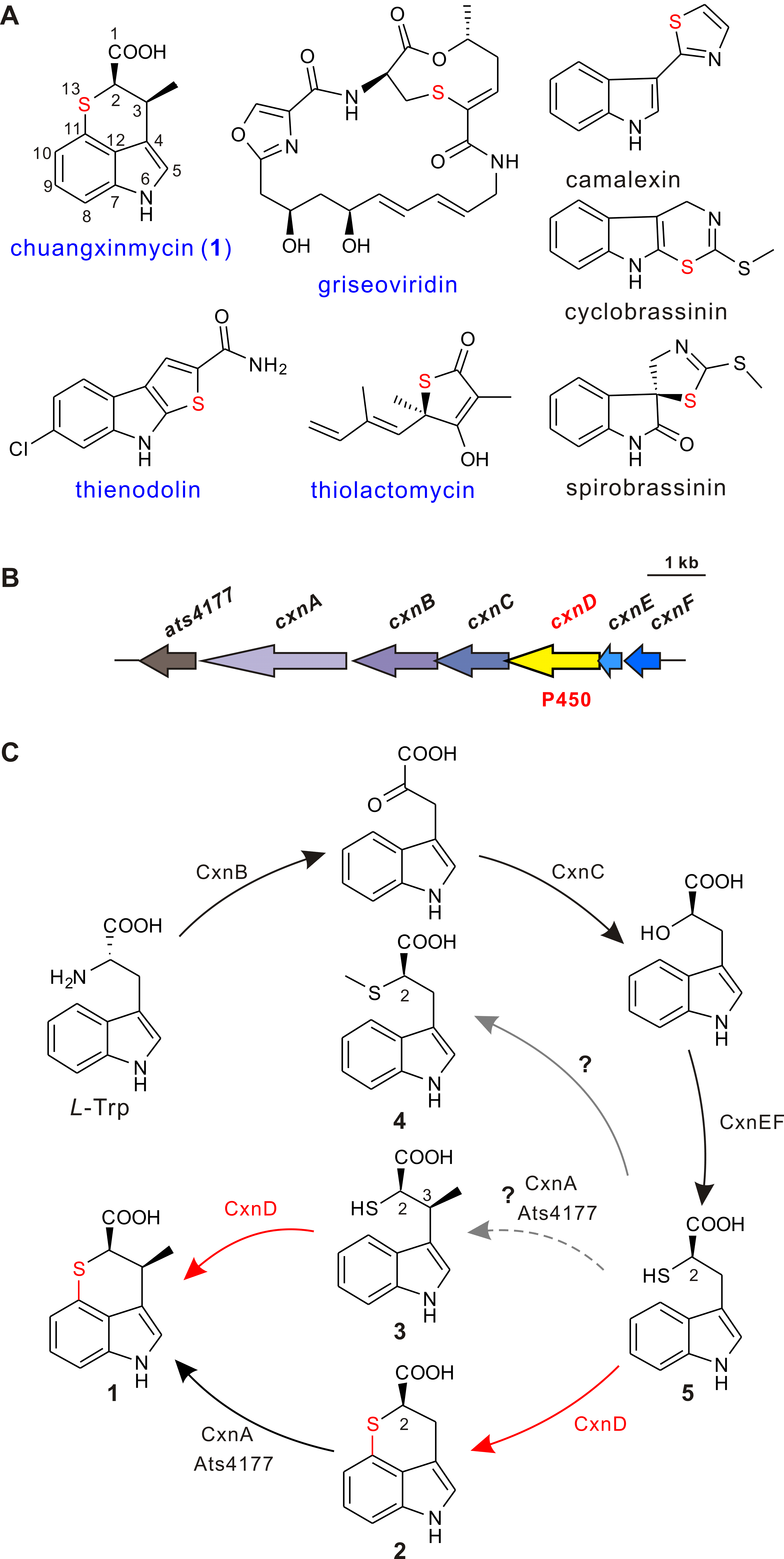
Chuangxinmycin, discovered in the 1970s by scientists from Institute of Antibiotics (now Institute of Medicinal Biotechnology), Chinese Academy of Medical Sciences, is a secondary metabolite containing an indole-dihydrothiopyran heterocyclic skeleton isolated from Actinoplanes tsinanensis CPCC 200056. Its biosynthetic gene cluster was located and cloned in 2017 by Hong’s and Wu’s labs ( Acta Pharm Sin B , 2017). And based on the product of cxnD -disruption mutant, an intra-molecular C-S bond formation by the cytochrome P450 CxnD was proposed to generate the tricyclic dihydrothiopyrano[4,3,2- cd ]indole skeleton. In the present research, they constructed the recombinant S. coelicolor M1152 which contained an incomplete cxn cluster without CxnD and methylation system CxnA and Ats4177 (i.e., CxnBCEF), isolated and identified the intermediate 4, suggesting that the compound 5 is the authentic substrate for CxnD catalysis in chuangxinmycin biosynthesis. Through in vivo feeding and in vitro enzyme assays, they demonstrated that CxnD can catalyze intra-molecular C( sp2 )-H thiolation of 3 and 5 to generate 1 and 2, respectively. Besides, 2R -5 was determined as the optimal substrate for CxnD.
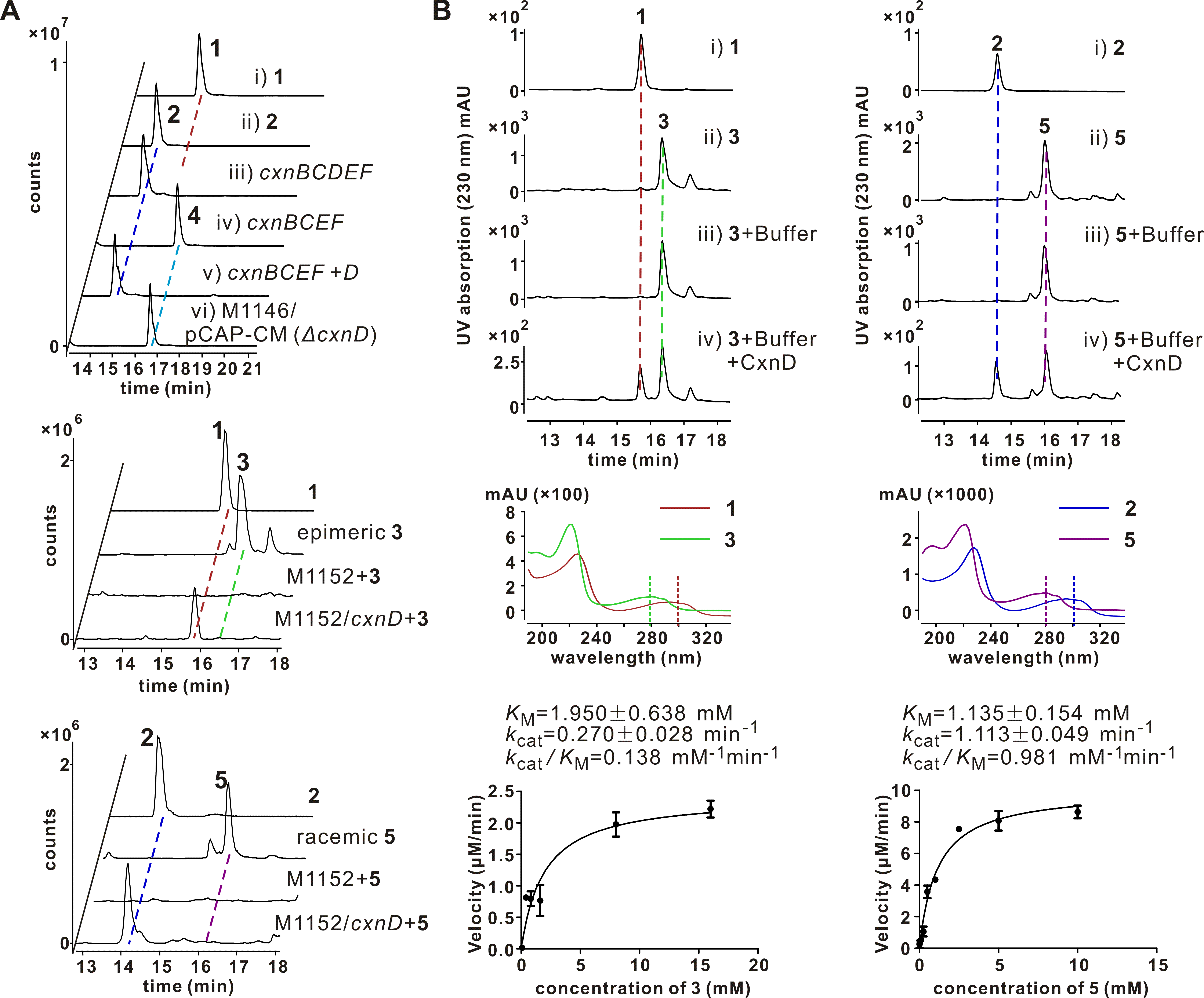
To further understand the structural basis of CxnD catalyzing the intra-molecular C-S bond formation, they solved CxnD (CxnD with a C28S replacement) crystal structure. Specifically, CxnD/C28S apoprotein and its complexes with 2, 3, or 4 were tried for crystallization. Fortunately, a high-quality CxnD/C28S with 4 needle crystal was obtained. The CxnD/C28S crystal structure was resolved at resolution 2.0 Å (PDB ID 6KFW). A molecular docking of 2R -5 into CxnD/C28S by AutoDock Vina was conducted to predict the binding mode of the authentic substrate. The active site cavity and binding mode of 2R -5 is similar to that of 4 in CxnD, and interactions between 2R -5 and CxnD to hold the substrate at its orientation and position in the active site cavity were revealed.

Site-directed mutations of CxnD were conducted to verify the importance of amino acids potentially involved in substrate binding. The substantially decreased/lost activity of T239A/P mutants, with similar binding affinity to 5 as wild-type CxnD, suggested the importance of the conserved T239 in the catalytic activity, which was also the same for many other P450s. Especially, K238E, which restored the conserved acidic amino acid (D/E) in many P450s, exhibited no activity, suggesting K238 may not involve in the proton delivery system. This result is in agreement with the necessity of K238 in the interaction with the carboxyl group of the substrate, which might account for the unique reaction mechanism of CxnD.
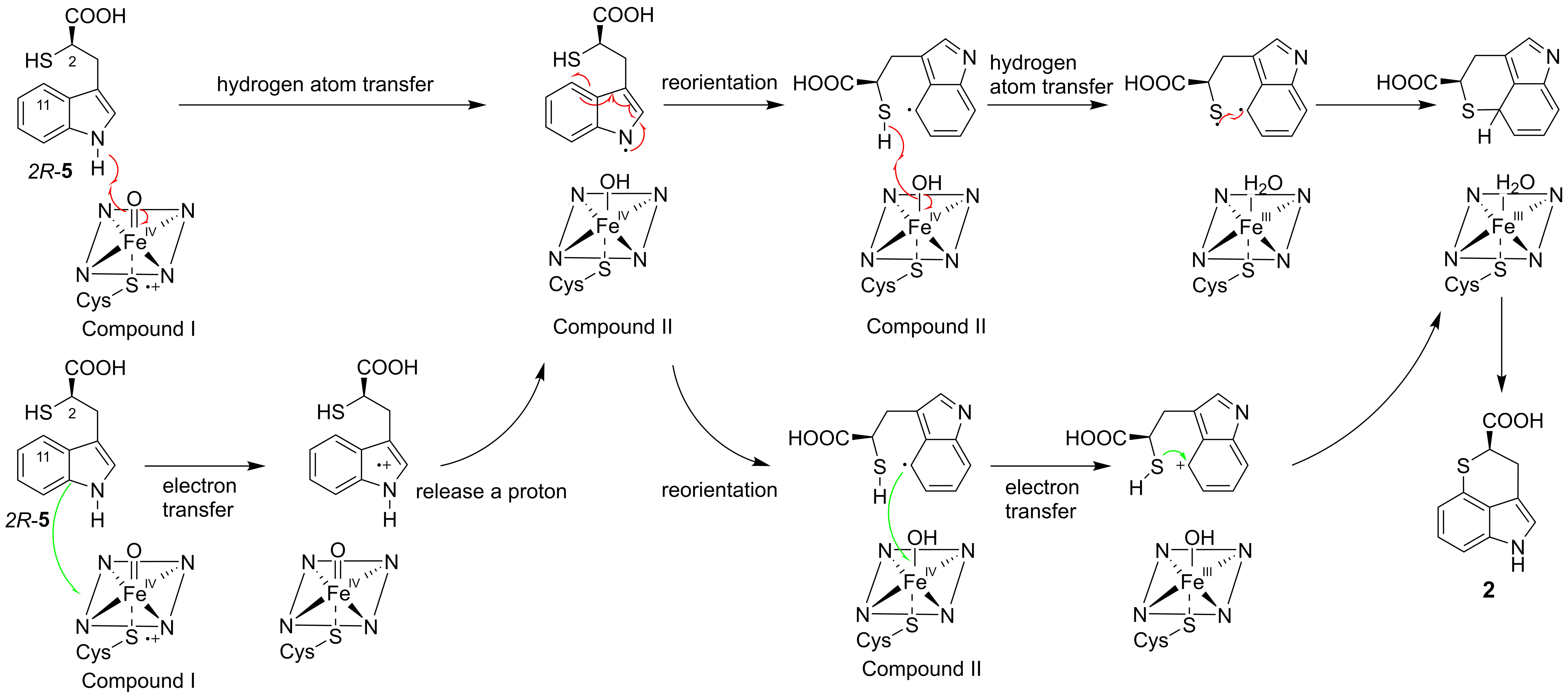
In summary, the genetic, biochemical and crystallographic studies of CxnD have provided the first insight into the molecular basis of intra-molecular C( sp 2)-H thiolation in the biosynthesis of the tricyclic indole- S -hetero skeleton of chuangxinmycin: The reaction is initiated by abstraction of hydrogen atom from the imino group of 2 R -5 or one electron from the indole ring by a ferryl-oxo species (Compound I). By repositioning and stabilizing the initial radical intermediate in the active site, an indole C-11 radical was formed. The hydrogen atom from thiol group was then abstracted by the ferryl-hydroxo species (Compound II) to generate the S radical, or the C-11 indole radical is directly oxidized by Compound II to yield a carbocation. A covalent bond is then formed between C-11 and S-13.
To our knowledge, this is the first biochemically and structurally characterized P450-mediated C-S bond formation in the biosynthesis of microbial secondary metabolites, broadening our understanding of C-S bond formation in natural products. This work is supported by National Key Research and Development Program of China (2018YFA0902000) and National Natural Science Foundation of China (81872780, 81803410). Dr. Yuanyuan Shi is the first author. Prof. Bin Hong and Prof. Linzhuan Wu are the co-corresponding authors.




